New research has revealed an additional link between the immune system and blood clots. This could result in improved treatment of critical illnesses.
SuPAR Identifies Patients at High Risk of Blood Clot Formation
A study from a COVID-19 cohort reveals an additional link between the immune system and blood clots, which could improve the treatment of critical illnesses.
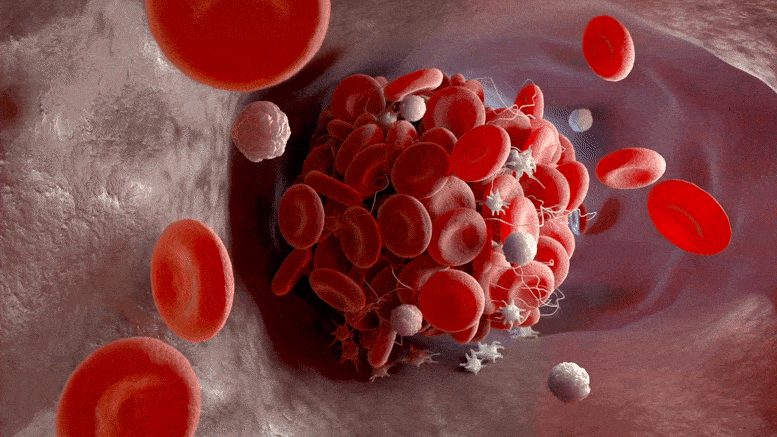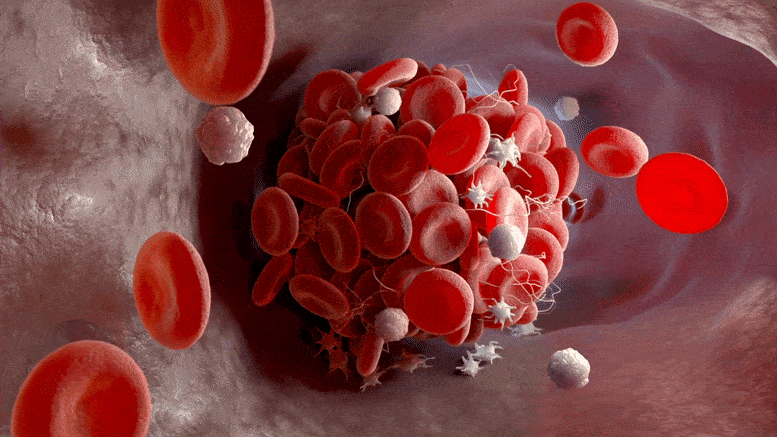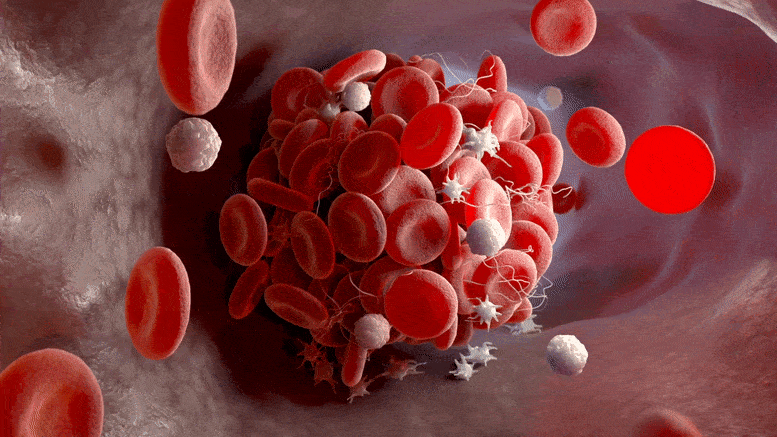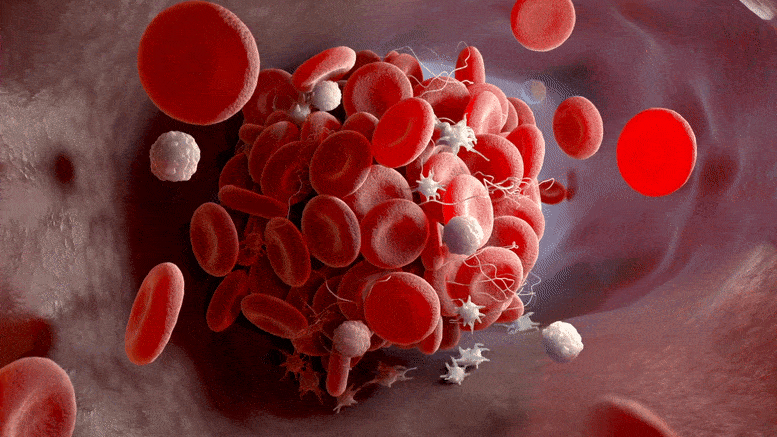
Blood clots are thought to occur in as many as a third of patients hospitalized with COVID-19. In many cases, these clots can be deadly, such as pulmonary embolisms—blood clots that travel to the lungs. In fact, in nearly one-third of patients with COVID-19, these clots led to death.
An abnormal immune response is thought to be the primary driver of severe COVID-19. One protein, called soluble urokinase plasminogen activator receptor, or suPAR, circulates in the blood and originates from immune cells. It has been shown to play a major role in complications of COVID-19.
A team of researchers from around the world, including Salim Hayek, M.D., Medical Director of the University of Michigan Frankel Cardiovascular Center Clinics, and Shengyuan Luo, M.D., internal medicine resident physician at Rush University Medical Center, have been studying suPAR and its relationship to critical outcomes in COVID-19 cases.
Researchers found that higher suPAR levels were associated with an increased risk of blood clot formation in a publication by the International Study of Inflammation in COVID-19, a multinational observational study of patients hospitalized for COVID-19.
Their new findings, published today (August 4, 2022) in the Journal of the American Heart Association, suggest that suPAR levels in hospitalized COVID patients were associated with venous thromboembolism including pulmonary embolism independently from a marker of blood clot formation called D-dimer.
“Traditionally, clinicians utilize D-dimer, a blood clot breakdown product, to assess VTE activity,” Luo said. “However, this marker has proven to be less predictive in COVID-19, as blood clot formation is in large part caused by a uniquely abnormal immune response to the virus.”
The researchers, therefore, conceived that combining suPAR, a marker of the immune system, and D-dimer could improve the reliability of determining who is at high or low risk of blood clot formation among COVID hospitalized patients.
“Even before the pandemic, before COVID-19, we had this idea about suPAR,” Hayek said. “We were seeing levels of the suPAR marker as the strongest risk factor for bad outcomes in other viral infections and in heart and kidney disease.”
When scientists discovered the severity of blood clots forming in COVID-19 patients early in the pandemic, they turned to suPAR for more insight. Earlier studies showed that suPAR levels were three to five times higher in COVID-19 patients and often associated with disease complications.
“We had previously shown that patients with high suPAR levels are at much higher risk of death, kidney injury, respiratory failure needing mechanical ventilation and now venous thromboembolism,” said Hayek.
Study findings
In the study, scientists compiled data from 1,960 adults who were hospitalized for COVID-19 and who had their suPAR levels measured at the time of hospital admission. All patients were monitored until they were either discharged, or in some cases, until death.
Important attributes for patients in this study included: age, sex, race, and body mass index. Additional medical conditions assessed upon admission included: diabetes, congestive heart failure, hypertension, stroke, and other critical cardiology and inflammatory diseases.
Researchers measured D-dimer and suPAR levels over a 30-day period during patients’ hospitalizations and diagnosed VTE (deep vein thrombosis and pulmonary embolism) using ultrasounds of the lower extremities and scans of the lungs.
Results showed that VTE occurred in 163 patients, and of those, 65 patients developed deep vein thrombosis, 88 patients developed a pulmonary embolus, and 10 patients developed both. Patients who developed blood clots had suPAR levels nearly 50% higher than those who did not develop clots. And, when suPAR levels were combined with D-dimer, researchers could classify 41% of study participants to have low-risk for occurrence of VTE.
“There is a modest positive correlation between suPAR and D-dimer levels; they both tend to trend in the same direction,” explained Hayek.
Now that the association is made between suPAR levels and blood clot formation, clinicians could assess who is at high or low risk, which will help them decide what therapies to use to treat them. For example, someone at high risk could be given anticoagulant medications before blood clot formation.
Studying suPAR and its link to the immune system has positive implications for critical COVID-19 patients, and beyond.
“In the background, there’s been a lot of work showing that this molecule (suPAR) is doing something bad to the body when levels are high,” Hayek said. “Companies are developing drugs to target suPAR, and so we might be measuring this on a regular basis.”
Hayek is optimistic about preventing critical outcomes within COVID-19 and other infectious diseases, and what the Michigan Medicine COVID-19 Cohort and the International Study of Inflammation in COVID-19 have been able to accomplish since the start of the pandemic.
Current studies are underway to test anti-suPAR therapies in patients with COVID-19.
“In the next year or so, we might be able to impact critical care in several other populations with implications that go beyond COVID,” said Hayek.
Reference: “Soluble Urokinase Plasminogen Activator Receptor and Venous Thromboembolism in COVID-19” by Shengyuan Luo, MBBS, M.H.S; Alexi Vasbinder, Ph.D, RN; Jeanne M. Du-Fay-de-Lavallaz, M.D.; Joanne Michelle D. Gomez, M.D.; Tisha Suboc, M.D.; Elizabeth Anderson, M.P.H; Annika Tekumulla; Husam Shadid, M.D.; Hanna Berlin, M.D.; Michael Pan, M.D.; Tariq U. Azam, M.D.; Ibrahim Khaleel, M.D.; Kishan Padalia, M.D.; Chelsea Meloche, M.D.; Patrick O’Hayer, M.D.; Tonimarie Catalan, B.S.; Pennelope Blakely, B.S.; Christopher Launius, B.S.; Kingsley-Michael Amadi, B.S.; Rodica Pop-Busui, M.D., Ph.D.; Sven H. Loosen, M.D.; Athanasios Chalkias, M.D.; Frank Tacke, M.D.; Evangelos J. Giamarellos-Bourboulis, M.D., Ph.D.; Izzet Altintas, M.D.; Jesper Eugen-Olsen, Ph.D.; Kim A. Williams, M.D.; Annabelle Santos Volgman, M.D.; Jochen Reiser, M.D., Ph.D, 4 August 2022, Journal of the American Heart Association.
DOI: 10.1161/JAHA.122.025198
Funding: NIH/National Heart, Lung and Blood Institute, NIH/National Heart, Lung and Blood Institute, NIH/National Institute of Diabetes and Digestive and Kidney Diseases, NIH/National Institute of Diabetes and Digestive and Kidney Diseases, Frankel Cardiovascular Center COVID-19: Impact Research Ignitor


0 Comments