
Researchers have discovered a new signaling molecule that forces brown fat cells to consume more energy.
Typically, fat cells store energy. However, energy is lost as heat in brown fat cells, making brown fat a biological heater. This mechanism is consequently present in most mammals. In humans, brown fat keeps babies warm, and in adults, brown fat activation favorably correlates with cardio-metabolic health.
“Nowadays, however, we’re toasty warm even in winter,” explains Prof. Dr. Alexander Pfeifer from the Institute of Pharmacology and Toxicology at the University of Bonn. “So our body’s own furnaces are hardly needed anymore.
We also move far less than our predecessors do while consuming a diet that is becoming more and more energy-dense. Brown fat cells are poisoned by these three factors: They gradually stop functioning entirely and die away. On the other hand, the global population of individuals who are extremely overweight continues to rise. “Research groups around the world are therefore looking for substances that stimulate brown fat and thus increase fat burning,” says Pfeifer.
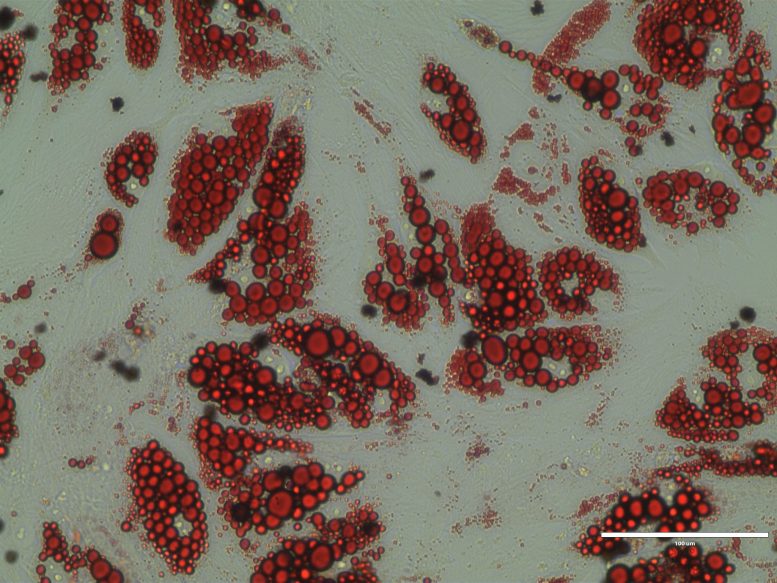
Human brown adipocytes, lipid stained red (RedO oil stain). Credit: Laia Reverte Salisa / University of Bonn
Dying fat cells boost the energy combustion of their neighbors
Together with a group of colleagues, the team at the University of Bonn has now identified a key molecule named inosine that is capable of burning fat. “It is known that dying cells release a mix of messenger molecules that influence the function of their neighbors,” explains Dr. Birte Niemann from Pfeifer’s research group. Together with her colleague Dr. Saskia Haufs-Brusberg, she planned and conducted the central experiments of the study. “We wanted to know if this mechanism also exists in brown fat.
The researchers, therefore, studied brown fat cells subjected to severe stress, so that the cells were virtually dying.
“We found that they secrete the purine inosine in large quantities,” Niemann says.
However, what was more intriguing was the way that intact brown fat cells reacted to the molecular cry for assistance: they were activated by inosine (or simply by dying cells in their vicinity). Inosine thus fanned the furnace inside them. White fat cells also converted to their brown siblings. Mice that were given a high-energy diet and inosine treatment at the same time stayed thinner than control animals and were protected from developing diabetes.
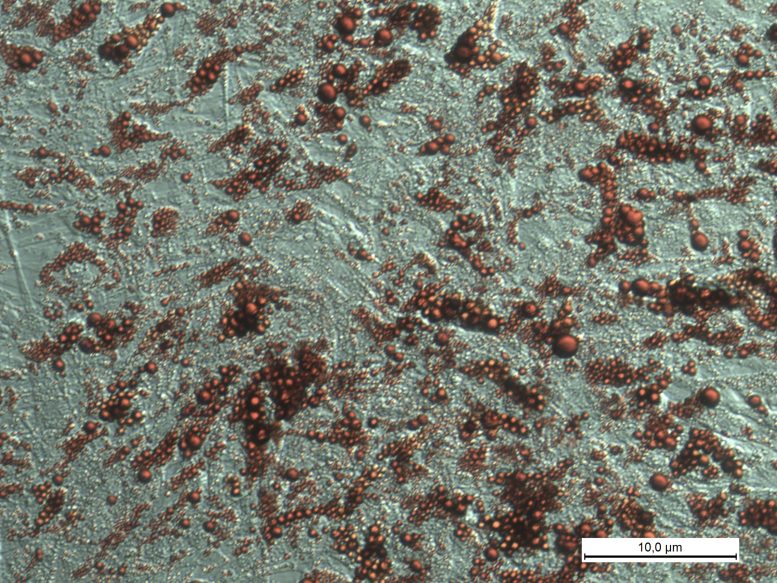
Another image of human brown adipocytes, lipid stained red (RedO oil stain). Credit: Thorsten Gnad / University of Bonn
The inosine transporter seems to play an important role in this context: this protein in the cell membrane transfers inosine into the cell, reducing the extracellular levels. As a result, inosine loses its ability to promote combustion.
The drug inhibits the inosine transporter
“There is a drug that was actually developed for coagulation disorders, but also inhibits the inosine transporter,” says Pfeifer, who is also a member of the Transdisciplinary Research Areas “Life and Health” and “Sustainable Futures” at the University of Bonn. “We gave this drug to mice, and as a result, they burned more energy.” Humans also have an inosine transporter. In two to four percent of all people, it is less active due to a genetic variation. “Our colleagues at the University of Leipzig have genetically analyzed 900 individuals,” Pfeifer explains. “Those subjects with the less active transporter were significantly leaner on average.”
These results suggest that inosine also regulates thermogenesis in human brown fat cells. Substances that interfere with the activity of the transporter could therefore potentially be suitable for the treatment of obesity. The drug already approved for coagulation disorders could serve as a starting point. “However, further studies in humans are needed to clarify the pharmacological potential of this mechanism,” Pfeifer says.
Neither does he believe that a pill alone will be the solution to the world’s rampant obesity pandemic. “But the available therapies are not effective enough at the moment,” he stresses. “We therefore desperately need medications to normalize energy balance in obese patients.
Reference: “Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine” by Birte Niemann, Saskia Haufs-Brusberg, Laura Puetz, Martin Feickert, Michelle Y. Jaeckstein, Anne Hoffmann, Jelena Zurkovic, Markus Heine, Eva-Maria Trautmann, Christa E. Müller, Anke Tönjes, Christian Schlein, Azin Jafari, Holger K. Eltzschig, Thorsten Gnad, Matthias Blüher, Natalie Krahmer, Peter Kovacs, Joerg Heeren and Alexander Pfeifer, 5 July 2022, Nature.
DOI: 10.1038/s41586-022-05041-0
The study was funded by the German Research Foundation and the National Institute of Health (USA).


0 Comments